
We are conducting the research on nanobiomaterials that function in the body. Nanobiomaterials can be designed to possess appropriate size and surface properties, allowing to control the distribution of substances in the body at a certain level. As a result, we can enhance the efficacy of medicines, reduce the side effects, or detect specific biological signals with high sensitivity.
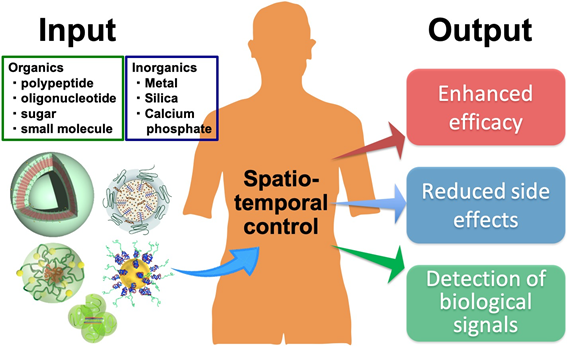
We are particularly focusing on biopharmaceuticals, such as nucleic acid drugs. Nucleic acid drugs have excellent potential for the treatment of many intractable diseases. However, their stability in vivo is not sufficient, and they are not efficiently uptaken by cells, making it difficult to proceed the clinical applications. These problems can be solved by "Nanomedicines" prepared using nanobiomaterials. Nanomedicines are designed to overcome the bottlenecks of a single drug or to amplify its efficacy. For example, nanomedicines can increase the efficiency of anticancer drug accumulation in tumor tissue with the reduced accumulation in normal tissues. It is also possible to promote the efficiency of uptake by specific cells.
We are creating new nanomedicines by designing nanobiomaterials that suit the purpose. Below, we introduce several research topics that are particularly focused on.
▶ The ultra-small nanomedicine for oligonucleotide delivery, termed Unit Polyion Complex
▶ In vivo CRISPR-Cas delivery system for genome editing, termed NanoEditor
▶ Silica-coated nanoparticles that protect fragile nucleic acid drugs
▶ Precise size-tunable polymer that penetrates the gap in the body, termed NanoRuler
Since the 1990s, a variety of cancer-targeted nanomedicines have been developed. Compared to the excellent results at the research level, clinical trials inn human cancer patients have been struggling. One of the reasons is that the tissue structure is considerably different between cancers at the research level (cancer-bearing mouse mode) and human cancers. It has been reported that human cancer tissues that grow over a longer period of time have greater heterogeneity, lower vascular density, and more fibrous tissue (called the interstitium). As a methodology to overcome these problems, we focus on downsizing of nanomedicines. Such a small-sized nanomedicine is envisioned to reach cancer cells by slipping through the small gaps that exist in cancer tissues and interstitium.
To design an ultra-small nanomedicine for siRNA delivery, we focused on the length and shape of the polymer. Indeed, we synthesized a Y-shaped block copolymer, as below. The polymer consisted of a two-armed biocompatible polymer (i.e. poly(ethylene glycol) (PEG)) and a cationic polypeptide. Specifically, the cationic polypeptide was polymerized to contain a positive charge equivalent to half the negative charge of siRNA (typically 40). In this way, a polyion complex was fabricated from two molecules of Y-shaped block copolymers and a single molecule of siRNA through their charge neutralization. This polyion complex was named "unit polyion complex (uPIC)" as the smallest unit of polyion complex because it selectively carries one molecule of siRNA. This uPIC had a steric repulsion effect produced by four molecules of long-chain PEG, and thus, its self-aggregation was substantially suppressed. The size of uPICs was maintained at ~18 nm in biological milieu. This size was less than one-fifth that of existing siRNA-loaded nanomedicines, such as lipid nanoparticles (~100 nm in diameter).
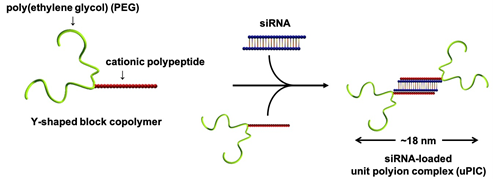
Ultimately, the siRNA/antisense oligonucleotide-loaded uPICs successfully accumulated in a fibrotic pancreatic cancer model, spontaneous pancreatic cancer model, and orthotopic brain tumor model, leading to the significant antitumor efficacy [Nat. Commun. 10, 1894 (2019)].
Additionally, we found that the structure of oligonucleotides is also critical for the in vivo stability of uPICs. Particularly, chemically modified and double-stranded oligonucleotides dramatically enhanced the half-life of uPICs in the blood after systemic administration [J. Control. Release 330, 812 (2021)].
Currently, a uPIC formulation loaded with cancer-targeted siRNA is in Phase I clinical trials. On the other hand, our laboratory is exploring new designs that further enhance the cancer targeting ability, as well as its applicability to diseases tissues other than cancer.

Recently, the genome editing by CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR Associated Proteins) has attracted much attention, as symbolized by the 2020 Nobel Prize in Chemistry. Compared to the other genome editing technologies, CRISPR-Cas has the great advantage of being highly flexible in target gene selection. Meanwhile, in order to apply CRISPR-Cas to medical treatment, we need to deliver CRISPR-Cas components (e.g. guide RNA and Cas protein or Cas-coded DNA/RNA) efficiently and selectively to the target cells. To date, viral vectors are mainly utilized to deliver those components into cells. However, there are concerns that viral formulations have risks of immunogenicity and mutagenicity, and that they will be extremely expensive. Therefore, we are exploiting a CRISPR-Cas delivery system using artificial polypeptide as an alternative. Specifically, we are designing a series of polypeptides with varying acid dissociation constants and hydrophobicity, and constructing Cas-coded mRNA-encapsulated nanomedicines, termed NanoEditor. When the optimized NanoEditor was administered intraventricularly or intrathecally into Ai9 mice, we confirmed that the genome editing was induced in a wide range of ventricular ependymal tissues [ACS Cent. Sci. 5, 1866 (2019)].
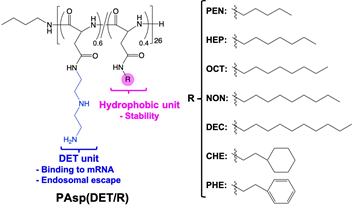
Our mRNA delivery technology is currently developed for the treatment of intractable diseases, such as Alzheimer's disease and muscular dystrophy.
In 2020, the pandemic of coronavirus disease 2019 (COVID-19) stalled various activities all over the world. To overcome this difficult situation, messenger RNA (mRNA) drugs have been successfully developed as a vaccine. Currently, mRNA drugs are further developed for the treatments of cancer and genetic diseases. However, mRNA is very susceptible to enzymatic degradation and is readily inactivated after administration into the body. Therefore, to maximize the therapeutic potential of mRNA drugs, the methodology to deliver intact mRNA from the site of administration to the target cells is strongly desired.
To this end, we develop a stable mRNA nanocarrier using organic/inorganic hybrid materials. Specifically, a silica-coated polyion complex, termed PicSil, has been fabricated by tightly encapsulating a polyion complex (PIC) with silica layer, as illustrated below. Silica consists of a network of silanol groups (Si-OH) and their condensed structure, siloxane bonds (Si-O-Si), and this network acts as a barrier disturbing the access of ribonucleases. We found that the amount of intact mRNA after incubation in ribonuclease-containing media was dramatically increased by silica-coating. Additionally, PicSil was efficiently uptaken by cultured macrophages, resulting in much higher mRNA expression compared to non-coated controls. These results demonstrate the strong potential of PicSil for macrophage-targeted mRNA delivery.
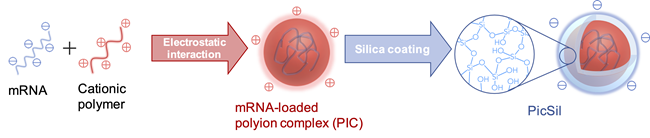
PicSil can also be applied for the delivery of other nucleic acids, such as plasmid DNA (Biomaterials 31 4764 (2010))and siRNA (J. Biomater. Sci. Polym. Ed. 28 1109 (2017), Biomaterials 34 562 (2013), ACS Nano 6 6693 (2012)).
The University of Tokyo, Department of Materials Engineering, Bld.#4, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656












